Home » Health News »
Genomic region for impaired memory function and anxiety in Down syndrome identified
A UCL-led research team has, for the first time, identified a specific region of chromosome 21, which causes issues with memory function and anxiety in a mouse that models Down syndrome, a finding that provides valuable new insight into the condition in people.
Most people have 46 chromosomes in each cell, divided into 23 pairs: people with Down syndrome (DS) have an extra copy of chromosome 21, which carries over 200 genes.
In this study, published in iScience, researchers at UCL, supported by Cardiff University and the Francis Crick Institute, used mouse models to highlight a genetic region carrying genes responsible for memory and behavior. The genes on human chromosome 21 are also found in mice, although they are dispersed onto three different mouse chromosomes; this includes mouse chromosome 10.
The research builds on a previous study by the same team, which examined the areas of chromosome 21 responsible for memory and decision-making problems in male mice that modeled Down syndrome. It also considers the genomic region linked to anxiety for the first time.
Anxiety is one of the most common mental health concerns in individuals with Down syndrome.
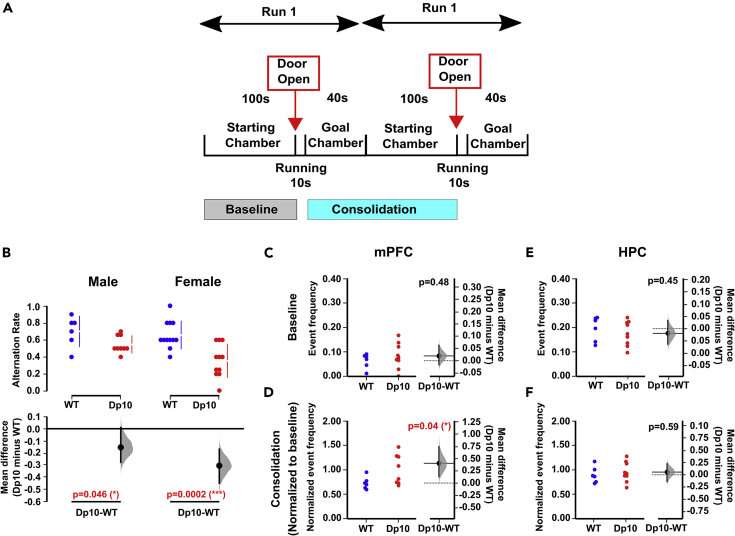
In the new study, the team looked at the effect of extra genes from mouse chromosome 10 that are the same as those on human chromosome 21, on anxiety and memory—and also considered female mice, to take into account gender.
To do this, mice that had been genetically modified to carry an extra copy of 37 distinct genes from mouse chromosome 10 were then compared to a control group of typically developing mice from the same litter.
During navigation tests, where mice needed to negotiate a simple ‘left-right’ T-maze, each group was measured for both memory and decision-making ability.
During these tests, the electrical activity in brain regions important for memory and emotional regulation (the hippocampus and medial prefrontal cortex) was also monitored using a measure known as Local Field Potential (LFP).
The researchers found that the genetically modified mice (‘Dp(10)2Yey’ mice) had worse short-term memory than the control group of mice. They also had irregular brain circuitry (signals) in the hippocampus—a brain region which is known to be crucial for memory.
This was the same for both male and female mice—showing that gender did not impact the outcome.
To test anxiety-like behavior, the researchers tested the reaction of the mice to being in a new environment.
The results found that the genetically modified mice were slower, moved less and spent more time on the periphery of the new environment, compared to in a familiar location—indicating anxious behavior.
Co-corresponding author, Professor Elizabeth Fisher (UCL Queen Square Institute of Neurology), said, “Down Syndrome is the most common form of intellectual disability. It arises from having three copies of chromosome 21 and is a genetic disorder in that some of the genes on this chromosome must produce the features of Down syndrome.”
“We wanted to investigate the characteristics of cognition in Down syndrome to understand which processes and genes are important.”
Co-corresponding author, Professor Matthew Walker (UCL Queen Square Institute of Neurology), added, “Chromosome 21 is made up of hundreds of genes but we only worked with a mouse with 37 of them duplicated. By whittling it down to very few genes, we can show which ones are important for the circuits in the brain and how they relate to memory and behavior in Down syndrome.”
The researchers hope that the findings could eventually lead to a therapy to aid cognition in Down syndrome.
Lead author, Dr. Pishan Chang (UCL Queen Square Institute of Neurology), said, “In this study we looked at two areas of the brain important for cognitive function. While the research is currently in mice, it allows us to build up a step-by-step picture of genes in this area and how they might change neuron architecture and function.”
“In the future this could help develop a strategy to relieve these features. For, by knowing which genes to target we may be able to consider solutions such as gene therapy or drugs in order to correct abnormal function. And eventually this may allow people with Down Syndrome to live more independent lives.”
More information:
Phillip M. Muza et al, Cognitive impairments in a Down syndrome model with abnormal hippocampal and prefrontal dynamics and cytoarchitecture, iScience (2023). DOI: 10.1016/j.isci.2023.106073
Journal information:
iScience
Source: Read Full Article



