Home » Health News »
Researchers develop deep learning model to predict adverse drug-drug interactions
Prescriptions for multiple drugs, or polypharmacy, is often recommended for the treatment of complex diseases. However, upon ingestion, multiple drugs may interact in an undesirable manner, resulting in severe adverse effects or decreased clinical efficacy. Early detection of such drug-drug interactions (DDIs) is therefore essential to prevent patients from experiencing adverse effects.
Currently, computational models and neural network-based algorithms examine prior records of known drug interactions and identify the structures and side effects they are associated with. These approaches assume that similar drugs have similar interactions and identify drug combinations associated with similar adverse effects.
Although understanding the mechanisms of DDIs at a molecular level is essential to predict their undesirable effects, current models rely on structures and properties of drugs, with predictive range limited to previously observed interactions. They do not consider the effect of DDIs on genes and cell functionality.
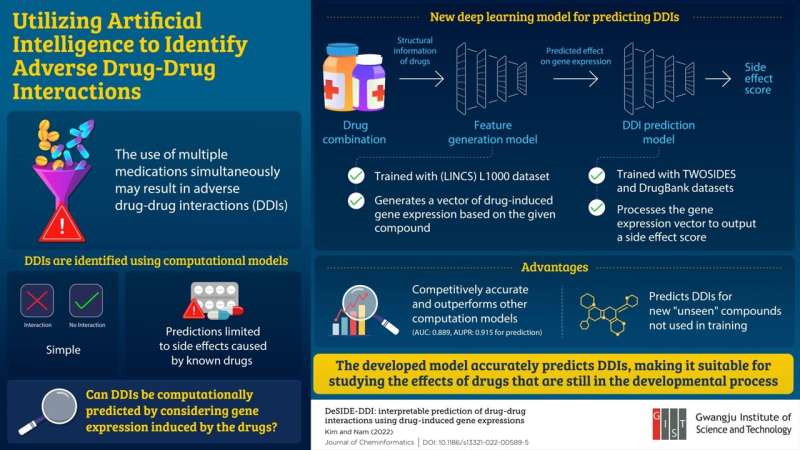
To address these limitations, Associate Professor Hojung Nam and Ph.D. candidate Eunyoung Kim from the Gwangju Institute of Science and Technology in South Korea developed a deep learning-based model to predict DDIs based on drug-induced gene expression signatures. These findings were published in the Journal of Cheminformatics on March 4, 2022.
The DeSIDE-DDI model consists of two parts: a feature generation model and a DDI prediction model. The feature generation model predicts a drug’s effect on gene expression by considering both the structure and properties of the drug while the DDI prediction model predicts various side effects resulting from drug combinations.
To explain the key features of this model, Prof. Nam explains, “our model considers the effects of drugs on genes by utilizing gene expression data, providing an explanation for why a certain pair of drugs cause DDIs. It can predict DDIs for currently approved drugs as well as for novel compounds. This way, the threats of polypharmacy can be resolved before new drugs are made available to the public.”
What’s more, since all compounds do not have drug-treated gene expression signatures, this model uses a pre-trained compound generation model to generate expected drug-treated gene expressions.
Source: Read Full Article



