Home » Health News »
Study finds lower risk of severe infection and hospitalization with belimumab compared to oral immunosuppressants
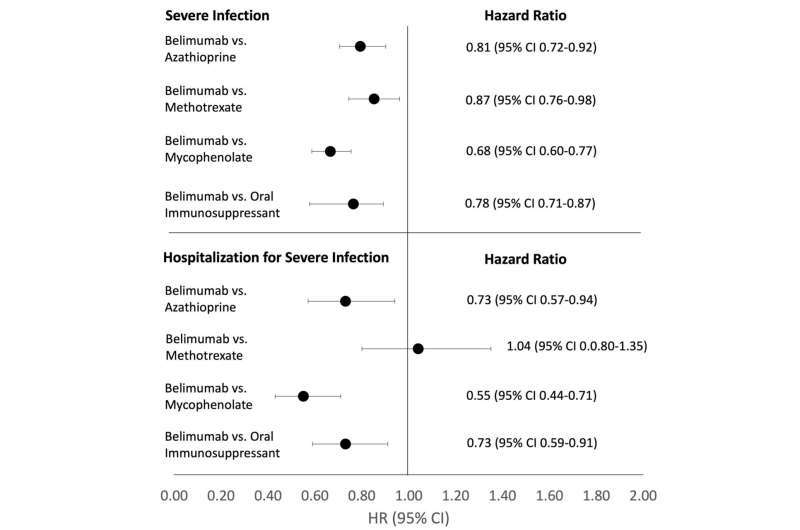
New research presented this week at ACR Convergence 2022, the American College of Rheumatology’s annual meeting, found that the biologic B-cell inhibitor belimumab was associated with a lower risk of severe infections and hospitalizations compared to nonbiologic immunosuppressants.
Until recently, belimumab was the only FDA-approved biologic for active non-renal systemic lupus erythematosus (SLE or lupus). Although infection is a major concern in treating SLE, there is little data on the infection risk for patients initiating belimumab compared to immunosuppressive medications such as azathioprine, methotrexate, and mycophenolate. The researchers undertook this study to better understand how medications used to treat lupus can contribute to infection risk.
Using observational data from TriNetX (a multi-center electronic health record database) the researchers identified adults aged 18 and older diagnosed with SLE but not lupus nephritis who began taking belimumab, azathioprine, methotrexate, or mycophenolate between 2011 and 2021.
They then designed three hypothetical trials to estimate the cumulative incidence and hazard ratios of severe infection and hospitalization due to severe infection comparing belimumab separately against azathioprine, methotrexate, and mycophenolate. In each comparison, patients had never used the comparator but could use one of the other drugs. For example, in the belimumab versus azathioprine trial, patients could use methotrexate or mycophenolate.
To replicate a randomized trial, the researchers used propensity score overlap weighting, a type of propensity score analysis.
“We used propensity score overlap weighting to approximate randomization of treatment assignments by balancing baseline covariants among patients in different arms of the study,” explains Emma Materne, MD, an internal medicine and pediatrics resident at Massachusetts General Hospital and the study’s lead author.
“Rather than adjust for individual covariants, each study subject has a propensity score calculated as the probability that they would receive the opposite treatment or intervention of interest, based on their set of covariants (age, race and ethnicity, SLE severity index, use of other SLE medications, chronic kidney disease and infection history, among others). This results in a target population that mimics the treatment groups in a randomized trial,” explained Dr. Materne.
The researchers compared 2,841 and 6,343 initiators of belimumab and azathioprine, 2,642 and 8,242 initiators of belimumab and methotrexate and 2,813 and 8,407 initiators of belimumab and mycophenolate, respectively.
After propensity score overlap weighting, all covariants were balanced in each comparison. The mean age was 45, 94% of patients were women, and 26-28% were Black.
In this large cohort of patients with non-renal SLE, belimumab was associated with a lower risk of severe infection and hospitalization due to severe infection compared to three oral immunosuppressants.
“The risk of infection and hospitalization should be taken into consideration when clinicians are initiating a new therapy for a patient. Our study shows that belimumab may have lower risks than oral immunosuppressants. It’s the first study to our knowledge comparing adverse outcomes in patients on belimumab compared to oral immunosuppressants in a head-to-head analysis. Our study design is in contrast to previous work that looked at outcomes with belimumab as an additive medication to other immunosuppressant therapy,” Dr. Materne says.
Dr. Materne notes that one of the strengths of the study is the inclusion of a large proportion of Black patients who are disproportionately affected by SLE. “We anticipate our findings apply across races and ethnic groups.”
More information:
Conference abstact
Source: Read Full Article



