Home » Medications »
Aimovig
01/31/2021 | Medications
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
AIMOVIG®
This medicine is subject to additional monitoring in Australia. This will allow quickidentification of new safety information. You can help by reporting any side effectsyou may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
solution for injection in pre-filled pen
erenumab
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about AIMOVIG. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking AIMOVIG against the benefits they expect it will have for you.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine.
You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What AIMOVIG is used for
AIMOVIG contains the active substance erenumab. It belongs to a group of medicines called anti CGRP antibodies (CGRP stands for calcitonin gene related peptide).
AIMOVIG works by blocking the activity of the CGRP molecule, which has been linked to migraine.
AIMOVIG is used to treat migraine. It is intended to reduce the number of days each month you have a migraine.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor’s prescription.
This medicine is not expected to affect your ability to drive a car or operate machinery.
Before you take AIMOVIG
When you must not take it
Do not take AIMOVIG if you have an allergy to:
erenumab
any of the ingredients listed at the end of this leaflet.
Allergic reactions can happen within minutes, but some may happen more than one week after using AIMOVIG.
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin
Do not give this medicine to a child or adolescent under the age of 18 years.
Safety and effectiveness in children younger than 18 years have not been studied in this age group.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
problems with your kidneys
problems with your liver.
Tell your doctor if you have or ever have had an allergic reaction to latex.
The pen used to inject AIMOVIG contains natural rubber latex within the cap.
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding.
Your doctor can discuss with you the risks and benefits involved.
AIMOVIG has not been studied in pregnant women. It is not known whether AIMOVIG could harm your unborn baby.
It is not known whether AIMOVIG passes into breast milk. Your doctor will help you decide whether you should stop breast feeding or stop taking AIMOVIG.
AIMOVIG contains sodium
AIMOVIG contains less than 1 mmol sodium (23 mg) per dose, this means it is essentially “sodium free” and should not affect a sodium controlled diet.
If you have not told your doctor about any of the above, tell him/her before you start taking AIMOVIG.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor if you are taking any medication to prevent migraines.
How to take AIMOVIG
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet. If your doctor has prescribed AIMOVIG with another migraine medicine, follow your doctor’s instructions on how to use these medicines together.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Each pen contains 70 mg or 140 mg AIMOVIG. The usual dose of Aimovig is 70 mg once every 4 weeks. Your doctor might also decide you need 140 mg once every 4 weeks. Take Aimovig exactly as instructed by your doctor.
70 mg/mL solution for injection
If your doctor prescribed the 70 mg dose you should have one injection once every 4 weeks in your abdomen, thigh or upper arm. If your doctor prescribed the 140 mg dose you should have two 70 mg injections once every 4 weeks. The second injection must be given immediately after the first.
140 mg/mL solution for injection
If your doctor prescribed the 140 mg dose, you will have one injection (subcutaneous) in your abdomen, thigh, or upper arm.
Make sure that you inject the entire contents of each pen.
How to take it
AIMOVIG is supplied in a pre-filled pen for single use. It is given as an injection under your skin (known as a subcutaneous injection). You or your caregiver can administer the injection into your abdomen, thigh or into the outer area of the upper arm (only if someone else is giving you the injection). Injection sites should be rotated and injections should not be given into areas where the skin is tender, bruised, red or hard.
Your doctor or nurse will give you or your caregiver training in the right way to prepare and inject AIMOVIG. Do not try to inject AIMOVIG until this training has been given.
Instructions for use of Aimovig pre-filled pen
Important: Needle is inside the pen
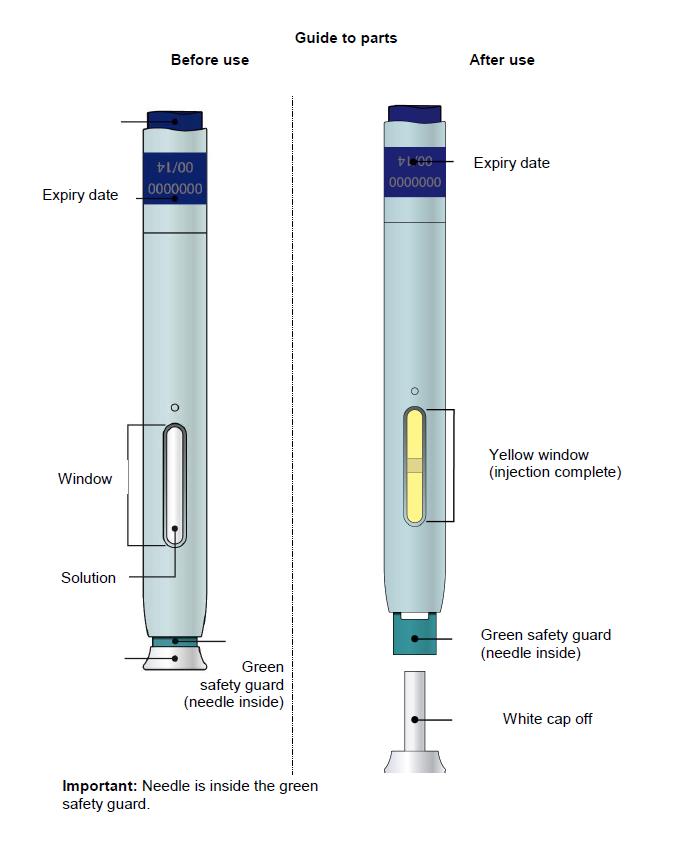
Single-Use Autoinjector/Pen 70 mg/mL
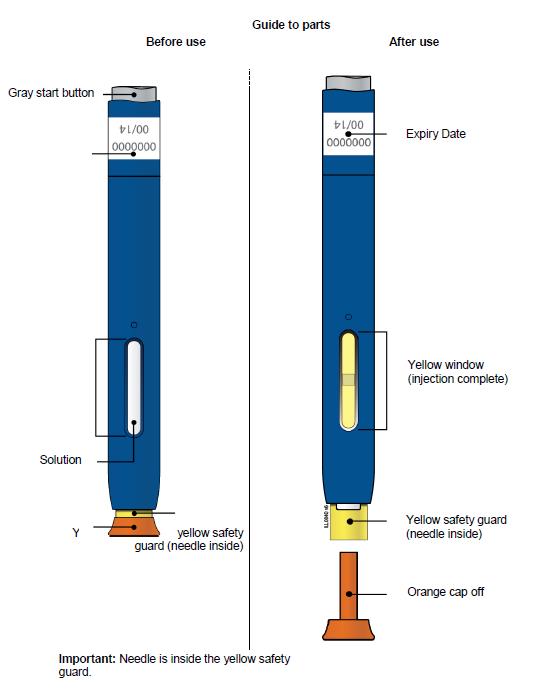
Single-Use Autoinjector/Pen 140 mg/mL
Step 1: Prepare
Your healthcare provider has prescribed a 70 mg or a 140 mg dose.
For a 70 mg dose, inject one autoinjector of 70 mg/mL.
For a 140 mg dose, inject either two autoinjectors of 70 mg/mL one after the other, or one autoinjector of 140 mg/mL if you were prescribed the 140 mg/mL formulation.
A) Carefully lift the pen(s) out of the carton
To avoid discomfort at the site of injection, leave the pen(s) at room temperature for at least 30 minutes before injecting.
Important:
Do not put the pen(s) back in the refrigerator once they have reached room temperature.
Do not try to warm the pen(s) by using a heat source such as hot water or microwave.
Do not leave the pen(s) in direct sunlight.
Do not shake the pen(s).
Do not remove the white cap from the pen(s) at this stage.
B) Inspect each pen
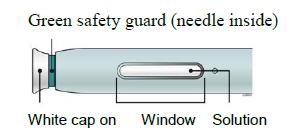
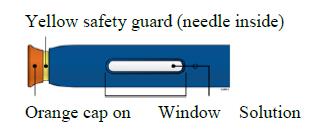
Make sure the solution you see in the window is clear and colourless to light yellow.
Important:
Do not use the pen if you notice that the solution contains easily visible particles, is cloudy or is distinctly yellow.
Do not use the pen if any part of it appears cracked or broken.
Do not use the pen if it has been dropped.
Do not use the pen if white or orange cap is missing or is not securely attached.
Do not use the pen after the expiry date stated on the label.
In all cases described above, use a new pen, and if you are unsure contact your doctor or pharmacist.
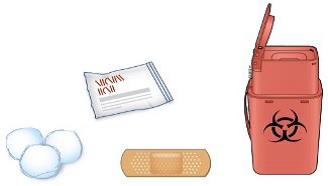
C) Gather all materials needed for the injection(s):
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the:
One or two new pen(s)
Alcohol wipes
Cotton balls or gauze pads
Adhesive plasters
Sharps disposal container

OR

D) Prepare and clean the injection site(s).
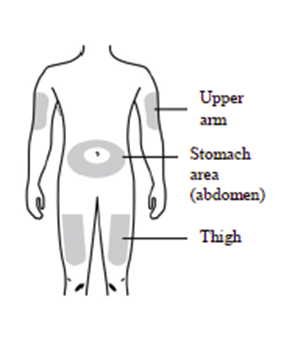
You can use any of the following injection sites:
Thigh
Stomach area (abdomen) (except for a 5 cm area around the navel)
Outer area of upper arm (only if someone else is giving you the injection)
Clean the injection site with an alcohol wipe and let the skin dry.
Choose a different site each time you give yourself an injection. If you need to use the same injection site, just make sure it is not the same spot on that site you used last time.
Important:
After you have cleaned the area, do not touch it again before injecting.
Do not choose an area where the skin is tender, bruised, red, or hard. Avoid injecting into a raised, thick, red or scaly skin patch or lesion, or areas with scars or stretch marks.
Step 2: Get ready
E) Pull the white or orange cap straight off, only when you are ready to inject. The injection must be administered within 5 minutes. It is normal to see a drop of liquid at the end of the needle or green or yellow safety guard.
Important:
Do not remove the white or orange cap from the pen until you are ready to inject.
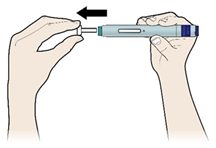
Do not leave the white or orange cap off for more than 5 minutes. This can dry out the medicine.
Do not twist or bend the white orange cap.
Do not put the white or orange cap back onto the pen once it has been removed.
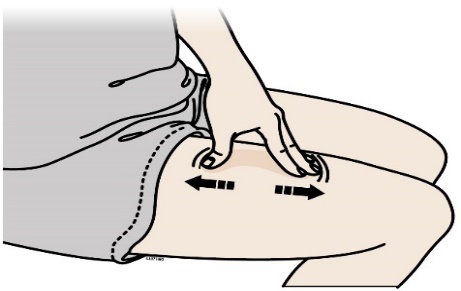
F) Stretch or pinch the injection site to create a firm surface.
Stretch method
Stretch skin firmly by moving your thumb and fingers in opposite directions, creating an area about five cm wide.
Or
Pinch method
Pinch skin firmly between your thumb and fingers, creating an area about five cm wide.
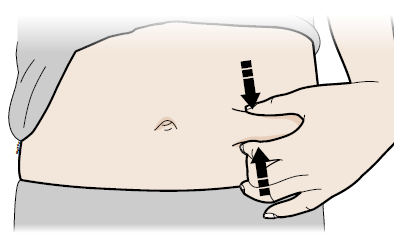
Important:
Keep skin stretched or pinched while injecting.
Step 3: Inject
G) Keep holding the stretch or pinch. With the white or orange cap off, place the pen on the skin at an angle of 90 degrees.
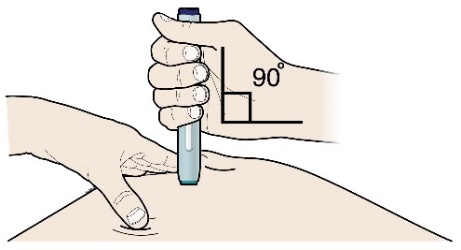
Important:
Do not touch the purple or grey start button yet.
H) Firmly push the pen down onto the skin until it stops moving.
Push down
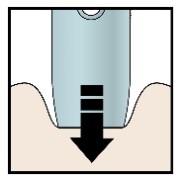
Important: You must push all the way down but do not touch the purple or grey start button until you are ready to inject.
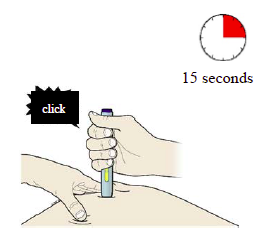
I) When you are ready to inject, press the purple or grey start button.
You will hear a click.
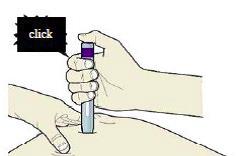
J) Keep pushing down on the skin. The injection could take about 15 seconds.
Important: Window turns yellow when injection is completed.
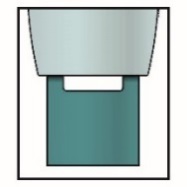
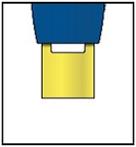
Note: After you remove the pen from the skin, the needle will automatically be covered by the green or yellow safety guard.
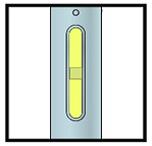
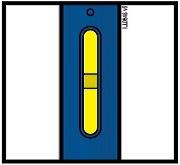
Important: When you remove the pen, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means you have not received a full dose.
Contact your doctor immediately.
Step 4: Finish
K) Discard the used pen and the white or orange cap.
Put the used pen in a sharps disposal container immediately after use. Talk to your doctor or pharmacist about proper disposal. There may be local regulations for disposal.
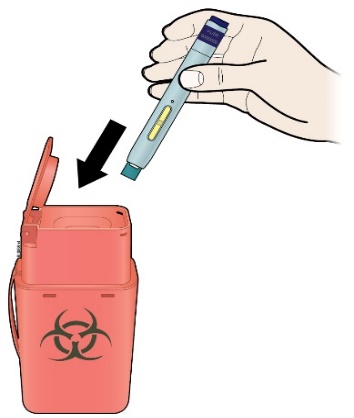
Important:
Do not reuse the pen.
Do not recycle the pen or sharps disposal container, or throw them into household waste.
Always keep the sharps disposal container out of the sight and reach of children.
L) Examine the injection site.
If there is a small sign of blood, press a cotton ball or gauze pad onto the injection site. Do not rub the injection site. Apply an adhesive plaster if needed.

If you have been prescribed a 140 mg dose using two 70 mg/mL pens, a second injection is required. Repeat all steps with the second pen to inject the full 140 mg dose.
When to take it
The usual dose of Aimovig is 70 mg once every 4 weeks. Your doctor might also decide you need 140 mg once every 4 weeks. Take Aimovig exactly as instructed by your doctor. Each pen contains 70 mg or 140 mg AIMOVIG.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
You should see your doctor after you have been using AIMOVIG for 8 to 12 weeks to discuss whether you should keep using AIMOVIG or whether your dose should be changed. You should continue to see your doctor every 3 to 6 months while taking AIMOVIG.
If you forget to take it
Take it as soon as you remember, and then contact your doctor, who will tell you when you should schedule your next dose. Follow the new schedule exactly as your doctor has told you.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice if you think that you or anyone else may have taken too much AIMOVIG. Do this even if there are no signs of discomfort or poisoning.
While you are using AIMOVIG
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking AIMOVIG.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you must not do
Do not take AIMOVIG to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Do not stop using Aimovig without talking to your doctor first. Your symptoms may return if you stop the treatment.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking AIMOVIG.
This medicine helps most people with migraine, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
constipation
itching
muscle spasms
injection site reactions, such as pain, redness and/or swelling where the injection is given
allergic reactions such as rash or swelling, or sometimes difficulty breathing.
The above list includes the more common side effects of your medicine. They are usually mild to moderate.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using AIMOVIG
Storage
Keep the pen in the outer carton until it is time to take it.
If you take the pen out of the carton it may not keep well.
Keep pens in a refrigerator (2°C – 8°C). Do not freeze. Do not shake.
After AIMOVIG has been taken out of the refrigerator, it must be kept at room temperature (up to 30°C) in the outer carton box and must be used within 14 days. Do not put AIMOVIG back in the refrigerator after it has reached room temperature.
Do not store AIMOVIG or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP.
The expiry date refers to the last day of that month.
Do not use this medicine if you notice that the solution contains easily visible particles, is cloudy or is distinctly yellow.
Disposal
AIMOVIG pen is for single use only.
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Do not throw away any medicines via wastewater or household waste.
Product description
What it looks like
AIMOVIG is a solution which is clear to opalescent, colourless to light yellow, and practically free from particles. Do not use the solution if you notice that it contains easily visible particles, is cloudy or is distinctly yellow.
Ingredients
AIMOVIG contains 70 mg or 140 mg of erenumab as the active ingredient.
It also contains the following inactive ingredients:
Sucrose
polysorbate 80
sodium hydroxide
glacial acetic acid
water for injections.
This medicine does not contain lactose, gluten, tartrazine or any other azo dyes.
This product contains natural rubber latex within the needle cap.
Sponsor
AIMOVIG is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
Web site: www.novartis.com.au
® = Registered Trademark
© Copyright
This leaflet was prepared in May 2020.
AIMOVIG 70 mg solution of injection in pre-filled pen AUST R 289959
*AIMOVIG 140 mg solution for injection in pre-filled pen AUST R 309205
*Not all presentations marketed.
(aimpen060520c.doc based on PI aim060520i.doc)
Source: Read Full Article


