Home » Health News »
Novobiocin attacks BRCA-mutated cancer cells from within and without, study shows
As the first clinical trial of the drug novobiocin is about to open for patients with cancers carrying BRCA gene mutations, new research at Dana-Farber Cancer Institute shows the drug poses a double threat to tumor cells.
As reported today in Nature Communications, the researchers found that in addition to taking aim at the inner workings of BRCA-mutated cells, novobiocin also incites an immune system attack on the cells. The findings suggest that even if novobiocin proves as effective as hoped in clinical trials, it may work significantly better if paired with drugs that magnify the immune response.
In January, the U.S. Food and Drug Administration approved novobiocin for a clinical trial in patients with cancers harboring mutations in the BRCA1 or BRCA2 genes. The trial, to be led by Dana-Farber investigators, is expected to start enrolling patients by June 1.
“Novobiocin is an inhibitor of POLθ, an enzyme critical to the survival of cancers with mutations in BRCA1 or BRCA2,” explains the study’s lead author, Jeffrey Patterson-Fortin, MD, Ph.D., of Dana-Farber. “From previous research, we knew that disabling POLθ with novobiocin killed BRCA1- or BRCA2-mutant cancers. Here, we wanted to examine the contribution of the immune microenvironment—the immune system cells within a tumor—to the anticancer effect of novobiocin.”
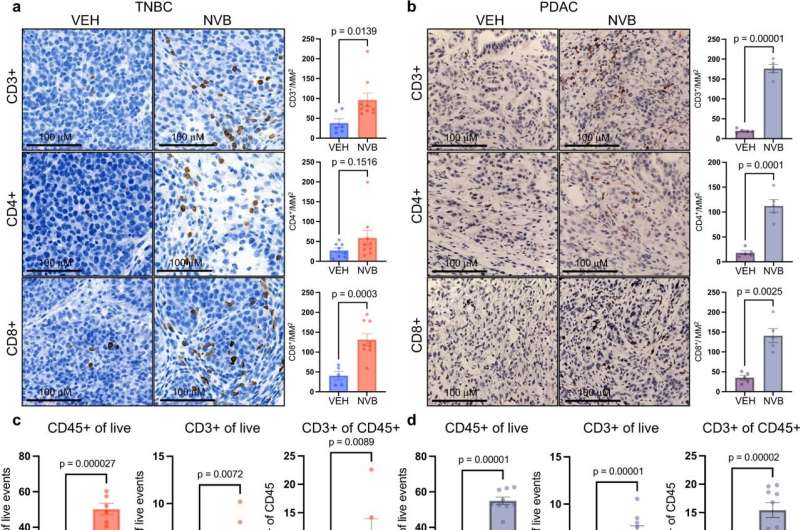
Using two tumor models—one of triple-negative breast cancer with BRCA1 mutations, and one of pancreatic cancer with BRCA2 mutations—researchers showed that novobiocin heightens the mayhem already occurring in cell division, with lethal consequences for tumor cells.
They found the drug causes tumor cells to become increasingly riddled with micronuclei—miniature nuclei that contain damaged fragments of chromosomes. The chromosome pieces slip easily through gaps in the micronuclei membranes and into the cells’ cytoplasm, activating a protein pathway known as cGAS/STING. The pathway causes disease-fighting CD8+ T cells to invade the tumors and go into attack mode.
To determine the extent to which this process is responsible for the tumor cell-killing effect of novobiocin, Patterson-Fortin and his colleagues depleted CD8+ T cells from the two tumor models and found the effect of novobiocin was sharply diminished. “This demonstrates that the stimulation of the immune system is a major part of novobiocin’s effectiveness, in addition to its direct effect on tumor cells,” says the study’s senior author, Geoffrey Shapiro, MD, Ph.D., of Dana-Farber.
One of the ways that the tumor cells adapt to the presence of novobiocin is by upping their production of the PD-L1 protein, which helps fend off a T-cell attack, Shapiro continues. This suggests that combining novobiocin with a PD-1-blocking agent could be more effective than novobiocin alone. Investigators hope to launch a clinical trial of the combination in the future.
More information:
Jeffrey Patterson-Fortin et al, Polymerase θ inhibition activates the cGAS-STING pathway and cooperates with immune checkpoint blockade in models of BRCA-deficient cancer, Nature Communications (2023). DOI: 10.1038/s41467-023-37096-6. www.nature.com/articles/s41467-023-37096-6
Journal information:
Nature Communications
Source: Read Full Article



