Home » Health News »
Aging impairs anti-tumor T-cell response via mitochondria dysfunction

MUSC Hollings Cancer Center researchers are finding solutions to the aging-related changes that reduce anti-cancer immunity. Besim Ogretmen, Ph.D., and colleagues found a novel link between aging, metabolism and anti-cancer T-cell function. Their work, published in Cell Reports, sheds light on an important pathway that cannot be ignored during cancer treatment.
Two broad questions in cancer research are: How can cancer treatments be improved, and what is the link between cancer and aging?
“We know that the protective T-cell response deteriorates with age. Mitochondrial function is now thought to be one of the central regulators of the aging process. Our experiments connected the dots with what was previously shown and highlighted some surprising and important pathways,” said Ogretmen, who is the SmartState Endowed Chair in Lipidomics and Drug Discovery.
All cells in the human body, except red blood cells, contain structures called mitochondria. Mitochondria are a cell’s central powerhouse—they produce the chemical energy necessary for survival. Ogretmen’s prior studies showed that a particular type of fatty molecule, called ceramide, causes mitochondrial dysfunction in cancer cells. In terms of cancer, mitochondrial dysfunction is thought to be a good thing since it weakens the cancer cells.
However, mitochondrial dysfunction might not be completely beneficial for cancer treatment. “We know that increased ceramide signaling is linked with aging, but it is important that we understand if this is also occurring in immune cells. Immunotherapy is becoming an increasingly important part of cancer treatment, so we must better understand the immune system in older people,” he said.
Aging stress produces the bioactive sphingolipid called ceramide. This lipid promotes mitochondrial dysfunction by a biological mechanism called mitophagy, where the broken mitochondria are selectively destroyed to kill cancer cells. However, scientists do not know if T-cells use this same pathway or if their immune functions are changed by ceramide signaling.
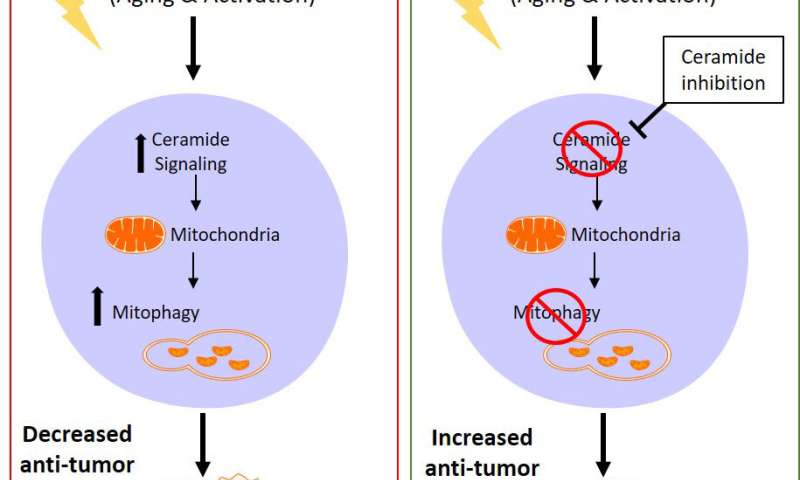
The results, which were surprising, showed ceramide signaling weakens anti-tumor T-cells. The data showed that:
- Aging decreases T-cell survival and function.
- Aging stress induces mitophagy in T-cells.
- Blocking certain ceramide molecules improves the anti-tumor function of aging T-cells.
“What this tells us is that ceramides need to be carefully controlled in a cancer setting,” said Ogretmen. This is particularly important because there are several FDA-approved drugs, such as FTY720 for multiple sclerosis, that increase ceramide signaling. Systemic increase of ceramide signaling in cancer patients may be harmful because it weakens the anti-cancer function of the patient’s immune system, he said.
Using multiple molecular methods, the team observed that T-cells from old mice, which were equivalent to approximately 50- to 60-year-old humans, had more ceramide accumulation in their mitochondria, which led to dysfunction. These cells had lower anti-tumor functions in cell culture and in cancer animal models. This biological pathway was confirmed by blocking ceramide signaling. Inhibiting ceramide metabolism by genetic or pharmacological means prevented mitophagy and restored the anti-cancer function of the aging T-cells, making them perform like younger counterparts.
“Our work was aided by a really strong team of collaborators and funding support from Hollings Cancer Center. The Team Science Award allowed us to generate this data, which has developed into something larger. We just resubmitted an NIH program project grant that focuses on the role of sphingolipids in T-cells and cancer,” he said.
The team will continue to piece together the biological pathways and processes to ensure that these findings are applicable to humans. The next step is to understand how aging induces ceramide stress. In general, stress can invoke dormant cancers, and this may be related to ceramide signaling.
Source: Read Full Article

