Home » Health News »
High magnetic field reduces side effects caused by anti-cancer drug imatinib
Researchers led by Prof. Zhang Xin at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences (CAS) combined the 9.4 T static magnetic field (SMF) with a commonly used anti-cancer drug imatinib mesylate on gastrointestinal-stromal-tumor-bearing mice. They found that the 9.4 T SMF not only enhanced the anti-tumor effect of imatinib, but also reduced the drug side effects on tissues and organs. In addition, it reduced depressive behavior in mice.
This work was published in the European Journal of Nuclear Medicine and Molecular Imaging.
SMF is the core part of magnetic resonance imaging (MRI), which plays an important role in improving the resolution of MRI images. 7 T MRI has been approved by the US Food and Drug Administration (FDA) for clinical diagnosis, and 9.4T MRI has been initially tested on healthy human volunteers.
However, since safety concerns are a prerequisite for high-field MRI applications, future applications of 9.4T MRI will benefit greatly from experiments in animal disease models. On the other hand, as the mainstream of anticancer therapy, the long-term use of targeted anticancer drugs often produces serious side effects and drug resistance, which greatly affects the clinical efficacy and the quality of life of patients.
In this study, scientists investigated the biosafety of 9.4 T SMF exposure in GIST tumor-bearing mice. They also evaluated the therapeutic potential of SMF alone or in combination with imatinib mesylate on tumor growth, side effects, and mental status in mice.
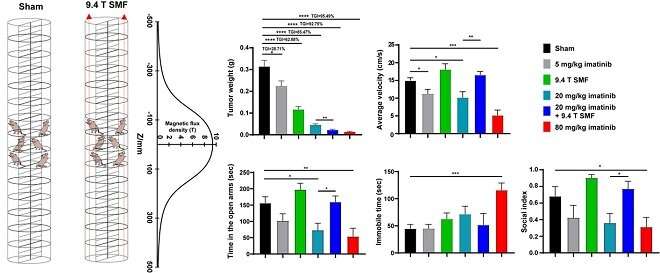
The results showed that the 9.4 T SMF treatment for 200 hours had good biosafety when it inhibited tumor growth by 62.88%, while combined with 20 mg/kg imatinib mesylate resulted in 92.75% tumor suppression.
This approximates the antitumor effect of high-dose (80 mg/kg) imatinib, but causes severe side effects, including significant weight loss, abnormal liver function, and depression in mice.
“The 9.4 T SMF treatment significantly reduced these imatinib-induced side effects, especially for the depressive behaviors,” said Bao Xun, first author of the study.
Source: Read Full Article



