Home » Health News »
Researchers review bacteriophage treatment
In a recent review published in Cell, researchers presented an overview of bacteriophage treatment, including mechanisms, types, design and applications of bacteriophage therapy.
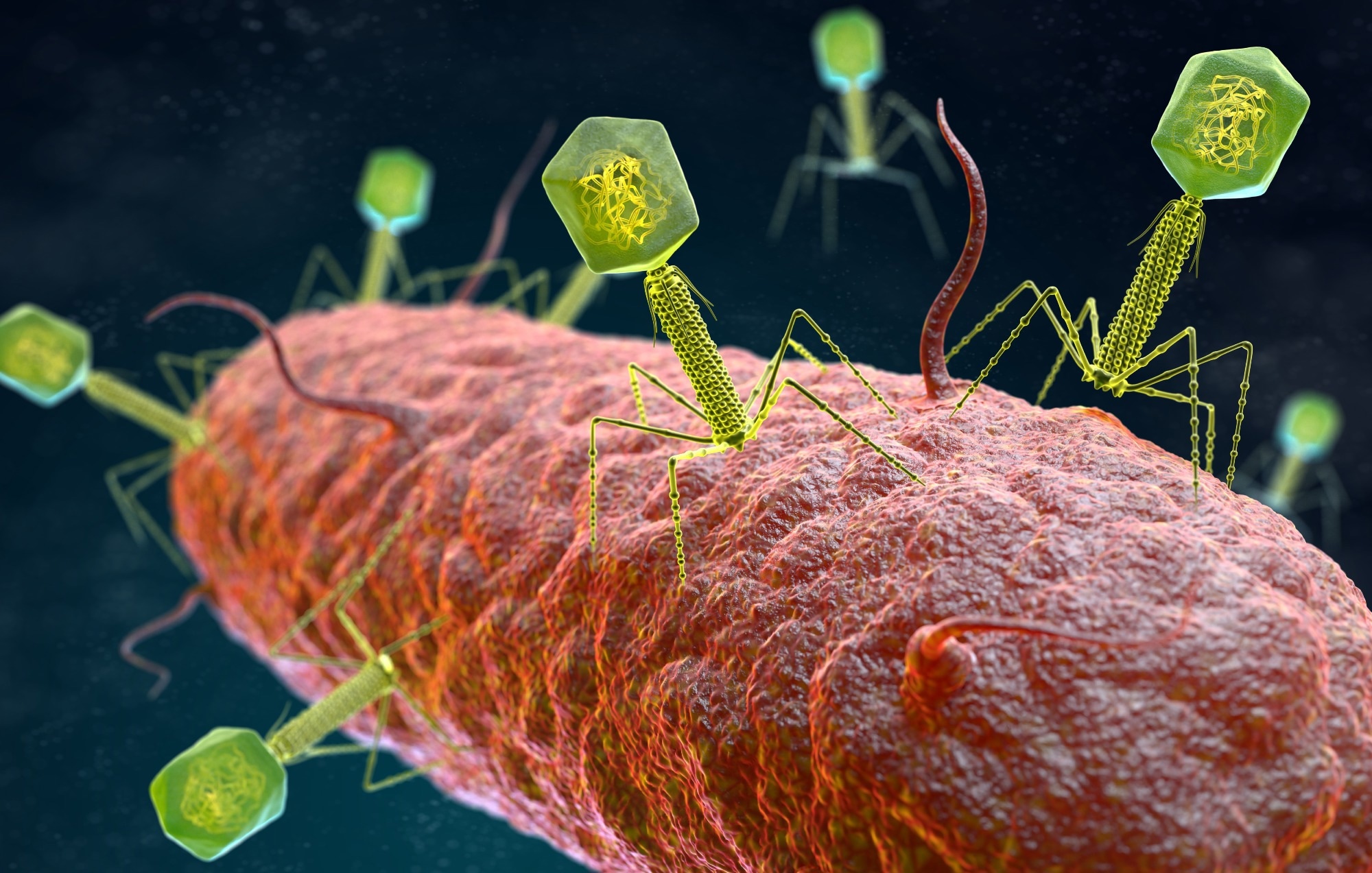
Antimicrobial resistance (AMR) has been associated with significant morbidity and mortality globally, especially among lower-income nations, and AMR incidence has increased in times of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The continual rise in AMR cases has reinvigorated research into substitutes, a promising avenue being bacteriophage treatment.
About the review
In the present review, researchers presented an overview of bacteriophage therapy, including the mechanisms, design, and applications.
Introduction to bacteriophage biology
Bacteriophages (derived from Greek, meaning ‘’bacteria eater’) are natural bacterial predators that evolved simultaneously with bacterial organisms for billions of years. Initially used by Felix d’Herelle in 1917 to treat bacterial dysentery among children, bacteriophage therapy has been extensively utilized for treating infections caused by bacteria among animals and humans, before penicillin use.
Bacteriophages are viruses that replicate within the host, have small-sized genomes, extensively utilize the machinery of the host to proliferate, and are specific to host cells. Bacteriophages require that bacteria express particular molecules on the surface to which the bacteriophage binds and that do not induce host defences which could inactivate the bacteriophage after entry.
The most commonly observed virion morphology comprises double-stranded deoxyribonucleic acid (dsDNA)-tailed bacteriophages, wherein deoxyribonucleic acid is located within the head or capsid which is connected to the tail. Infection commences by the tail end connecting to the cell wall of the bacteria and genomic insertion from the head/capsid in the cytoplasm via the cellular membrane. The proteinaceous head and tail do not enter the cells.
The majority of bacteriophages are either temperate or lytic. The lytic type of phages kills a very high percentage of bacterial cells they infect, and are, therefore, used as therapeutics. In the case of an infection, the lytic type of bacteriophages pursues one developmental program that involves gene expression in the initial phage, genomic replication, late bacteriolytic virion structure expression and gene assembly, packaged particle assembly, and lastly, lysis of bacteria. Phages are genomically highly diverse and phage genomes are tightly packed with overlapping protein-encoding and/or ribonucleic acid (RNA)-coding genes and are replete with small UKF (unknown function) genes.
Bacteriophage genomes are pervasively mosaics, with single genes (or gene subsets) in different genomic contexts in otherwise unrelated bacteriophages. Therapeutically bacteriophages include bacteriophages, Muddy and Maestro, used for treating infections by Mycobacterium abscessus infections and Acinetobacter baumannii, respectively.
Design and applications of bacteriophage therapy
Antibodies eBook

Phages may occur naturally or be genetically engineered. Environmental or naturally occurring phages can be found in places where their bacterial hosts are present, including lakes, oceans, animals, plants, and soil. Phages may be genetically engineered to allow for the programmed and functional arrangement of bacteriophage particles for greater penetrance of biofilms, targeting intracellular pathogens, or enhancing the pharmacodynamic and/or pharmacokinetic properties.
Phage genome engineering involves building engineered phages and recovering desired progeny from the parenteral strain pool. Homologous genomic engineering techniques are used in vivo with CRISPR (clustered regularly interspaced short palindromic repeats)-Cas (CRISPR-associated protein) system-based counterselection. The homologous genetic recombination technique involves recombining phage DNA with the homologous area on plasmid deoxyribonucleic acid. In vivo genetic engineering of phages involves recombining phage genome and electroporated products of polymerase chain reaction (PCR) analysis with homologous arms.
The BRED (bacteriophage recombineering of electroporated DNA) technique involves recombining co-electroporated phage deoxyribonucleic acid and PCR analysis products with homologous arms. Subsequently, RNA-guided ribonucleic acid nuclease (Cas13) or RNA-guided deoxyribonucleic acid nucleases (Cas9,12) counterselection is applied to selectively eliminate unedited bacteriophages.
Synthetic genome construction enables building genomes by design by combining fragments of bacteriophage genome amplified by PCR analysis and synthetic oligonucleotides. Synthetic bacteriophage genomes are conjugated into a vector by in vitro or yeast-based conjugation. Subsequently, the conjugated genomes are ‘rebooted’ by utilizing cell-free TXTL (transcription-translation) systems or appropriate bacteria.
Bacteriophage treatment has been used to treat infections in relation to implanted devices, and pulmonary infections, followed by prostatitis, burns, endocarditis, intra-abdominal infections, disseminated infections, urinary tract infections, osteomyelitis, and cutaneous infections. For humans, phage may be used as a vector to deliver vaccines or therapeutics, for biodefense to detect pathogens such as Bacillus anthracis or Yersinia pestis, prophylaxis during outbreaks (e.g., Vibrio cholera and Mycobacterium tuberculosis), and to groom microbiomes.
Phage may also be used in the veterinary treatment of Salmonella, E. coli, and Campylobacter infections and to replace antibiotics in livestock. Environmental applications include wastewater disinfection, food safety, and replacing antibiotics in aquaculture and agriculture.
To conclude, based on the review findings, phage therapy may be an effective alternative to antibiotic therapy to treat bacterial infections. Therapeutic bacteriophages must be lytic, kill the bacterial host efficiently, and be fully characterized to exclude side effects. Phage therapy is usually delivered intravenously and considered safe; however, adaptive immunological responses to the bacteriophage may compromise therapeutic efficacy.
- Steffanie A. Strathdee, Graham F. Hatfull, Vivek K. Mutalik, and Robert T. Schooley. (2023). Phage therapy: From biological mechanisms to future directions. Cell. doi: https://doi.org/10.1016/j.cell.2022.11.017 https://www.cell.com/cell/fulltext/S0092-8674(22)01461-1
Posted in: Medical Science News | Disease/Infection News
Tags: Acinetobacter, Acinetobacter Baumannii, Agriculture, Antibiotic, Antimicrobial Resistance, Bacteria, Bacteriophage, Biofilms, Campylobacter, Capsid, Cas13, Cas9, Cell, Cell Wall, Children, Cholera, Conjugation, Coronavirus, CRISPR, Cytoplasm, Disinfection, DNA, Dysentery, E. coli, Efficacy, Endocarditis, Food, Food Safety, Gene, Gene Expression, Genes, Genetic, Genetic Engineering, Genetic Recombination, Genome, Genomic, Homologous, in vitro, in vivo, Intracellular, Membrane, Morphology, Mortality, Nuclease, Oligonucleotides, Osteomyelitis, Palindromic Repeats, Pandemic, Penicillin, Plasmid, Polymerase, Polymerase Chain Reaction, Prophylaxis, Prostatitis, Protein, Research, Respiratory, Ribonucleic Acid, RNA, Salmonella, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Therapeutics, Transcription, Translation, Tuberculosis, Veterinary, Yeast

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article



