Home » Health News »
Type 2 diabetes accelerates brain aging and cognitive decline
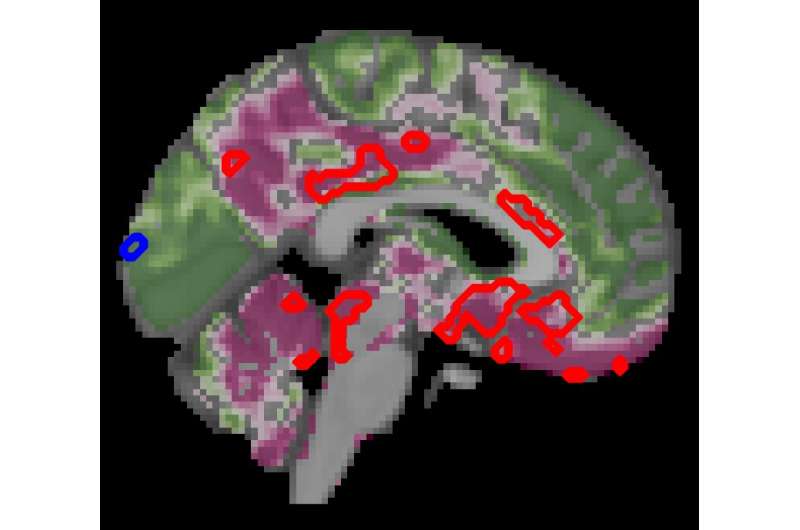
Scientists have demonstrated that normal brain aging is accelerated by approximately 26% in people with progressive type 2 diabetes compared to individuals without the disease, reports a study published today in eLife.
The authors evaluated the relationship between typical brain aging and that seen in type 2 diabetes, and observed that type 2 diabetes follows a similar pattern of neurodegeneration as aging, but one that progresses faster. One important implication of this finding is that even typical brain aging may reflect changes in the brain’s regulation of glucose by insulin.
The results further suggest that by the time type 2 diabetes is formally diagnosed, there may already be significant structural damage to the brain. Sensitive ways to detect diabetes-associated changes to the brain are therefore urgently needed.
There is already strong evidence linking type 2 diabetes with cognitive decline, yet few patients currently undergo a comprehensive cognitive assessment as part of their clinical care. It can be difficult to distinguish between normal brain aging that begins in middle age, and brain aging caused or accelerated by diabetes. To date, no studies have directly compared neurological changes in healthy people over the course of their lifespan with changes to those experienced by people of the same age with diabetes.
“Routine clinical assessments for diagnosing diabetes typically focus on blood glucose, insulin levels and body mass percentage,” says first author Botond Antal, a Ph.D. student at the Department of Biomedical Engineering, Stony Brook University, New York, US. “However, the neurological effects of type 2 diabetes may reveal themselves many years before they can be detected by standard measures, so by the time type 2 diabetes is diagnosed by conventional tests, patients may have already sustained irreversible brain damage.”
To define the impact of diabetes on the brain over and above normal aging, the team made use of the largest available brain structure and function dataset across human lifespan: UK Biobank data from 20,000 people aged 50 to 80 years old. This dataset includes brain scans and brain function measurements and holds data for both healthy individuals and those with a type 2 diabetes diagnosis. The team used this to determine which brain and cognitive changes are specific to diabetes, rather than just aging, and then confirmed these results by comparing them with a meta-analysis of nearly 100 other studies.
Their analysis showed that both aging and type 2 diabetes cause changes in executive functions such as working memory, learning and flexible thinking, and changes in brain processing speed. However, people with diabetes had a further 13.1% decrease in executive function beyond age-related effects, and their processing speed decreased by a further 6.7% compared to people of the same age without diabetes. Their meta-analysis of other studies also confirmed this finding: People with type 2 diabetes had consistently and markedly lower cognitive performance compared to healthy individuals who were the same age and similarly educated.
The team also compared brain structure and activity between people with and without diabetes using MRI scans. Here, they found a decrease in gray brain matter with age, mostly in a region called the ventral striatum—which is critical to the brain’s executive functions. Yet people with diabetes had even more pronounced decreases in gray matter beyond the typical age-related effects—a further 6.2% decrease in gray matter in the ventral striatum, but also loss of gray matter in other regions, compared with normal aging.
Together, the results suggest that the patterns of type 2 diabetes-related neurodegeneration strongly overlap with those of normal aging, but that neurodegeneration is accelerated. Moreover, these effects on brain function were more severe with increased duration of diabetes. In fact, progression of diabetes was linked with a 26% acceleration of brain aging.
Source: Read Full Article



