Home » Health News »
Updated treatment guidelines for drug-resistant TB
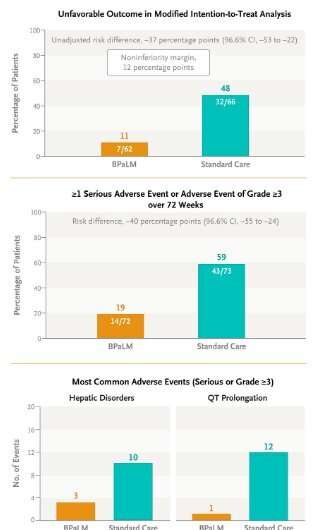
New World Health Organization (WHO) guidelines for the treatment of drug resistant tuberculosis (DR-TB) are shaping as a game changer for the estimated 500,000 people diagnosed with DR-TB each year, and the longer-term goal to end the global TB pandemic by 2035.
The guidelines are based on findings of a five-year trial called TB PRACTECAL which found that an all-oral treatment regimen using a combination of four drugs (bedaquiline, pretomanid, linezolid and moxifloxacin) over 24 weeks performed better than the existing standard of care requiring patients to take medications daily over nine to twenty months.
WHO also endorsed a second shorter regimen involving only three drugs which achieved high efficacy and safety results in the trial.
Burnet Institute Infectious Disease Specialist and member of the TB PRACTECAL Steering Committee, Dr. Philipp du Cros said the study findings have a “revolutionary potential” to expand access to treatments for DR-TB.
“The new regimens are particularly important for helping cover a key gap in the TB response in our region and globally,” Dr. du Cros said.
“Of the estimated 500,000 people who develop drug resistant TB each year, only about one in three is diagnosed and put on effective treatment, so the majority of people don’t have access to the correct treatment.
“We need simpler and shorter treatments that are better tolerated to be able to close that gap, and unless we close that gap, we’re not going to be able to end the TB epidemic.”
Dr. du Cros cautioned, however, that the new regimens alone will not be enough unless barriers—including the timely adoption of the new guidelines; access to the medications; effective surveillance; laboratory capacity to test for resistance; and support for patients including education and counseling—can be overcome.
“What we need is leadership from national TB programs to implement this early,” Dr. du Cros said.
“Some countries in our region have already been implementing operational research using this or similar short regimens, so they’re well placed to be able to scale up this new regimen.
“But we also need investment into research into how best to scale up and support patients to ensure this treatment reaches everyone.”
Dr. du Cros noted that while a six-month regimen is much shorter than the previous standard, it’s still long and demanding, and a high proportion of people with DR-TB are faced with catastrophic costs and the many resulting negative impacts for themselves, their families and communities.
Every year, about 10.6 million people globally develop tuberculosis including half a million with DR-TB at a rate that increased during the COVID pandemic. The Asia Pacific region bears a heavy burden, accounting for about two-thirds of all DR-TB cases globally.
More information:
Bern-Thomas Nyang’wa et al, A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis, New England Journal of Medicine (2022). DOI: 10.1056/NEJMoa2117166
Journal information:
New England Journal of Medicine
Source: Read Full Article



