ROCACTSC20420
pronounced (Act-tem-ra)
contains the active ingredient tocilizumab (rch)
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about Actemra pre-filled syringe for subcutaneous (under the skin) injection.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Actemra against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Actemra is used for
Actemra contains the active ingredient tocilizumab.
Actemra belongs to a group of medicines called anti-rheumatic agents. There are many different classes of anti-rheumatic agents. Actemra belongs to a class called monoclonal antibodies.
Monoclonal antibodies are proteins which specifically recognise and bind to other unique proteins in the body.
Actemra for subcutaneous injection is used to treat active moderate to severe rheumatoid arthritis (RA) and giant cell arteritis (GCA) in adults.
It is also used to treat active moderate to severe polyarticular juvenile idiopathic arthritis (pJIA) in children over 2 years of age and active systemic juvenile idiopathic arthritis (sJIA) in children and adolescents, aged 1 year and over.
Some of the signs and symptoms of these conditions are caused by the actions of a protein called interleukin-6 receptor (IL-6R).
Actemra works by binding and blocking IL-6R thereby helping to relieve some of the signs and symptoms of these conditions. For patients with RA, Actemra can also prevent damage occurring to your joints.
There are different types of medicines used to treat RA, GCA, pJIA and sJIA. Your doctor, however, may have prescribed Actemra for another purpose.
Ask your doctor if you have any questions about why Actemra has been prescribed for you.
Actemra is not addictive.
This medicine is available only with a doctor’s prescription.
When you must not use Actemra
Do not use Actemra if:
1.you have had an allergic reaction to Actemra or any ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
chest tightness, wheezing or difficulty breathing
severe dizziness or light-headedness
swelling of the face, lips, tongue, throat or other parts of the body with difficulty breathing
rash, itching or hives on the skin
2.you have had an allergic reaction to any other recombinant human or humanised antibodies or proteins that are of hamster origin
3.you have an active, severe infection
4.the package is torn or shows signs of tampering
5.the expiry date (EXP) printed on the pack has passed.
If you take this medicine after the expiry date has passed, it may not work as well.
If you are not sure if you should use Actemra, talk to your doctor.
Before you use Actemra
Tell your doctor if:
1.you have or develop any kind of infection
Actemra can reduce your body’s ability to respond to infections and may make an existing infection worse or increase the chance of getting a new infection. This may be important if you have diabetes or diverticulitis (which increase your risk of infection).
Your doctor will perform blood tests before you are given Actemra to determine if you have low white blood cell or platelet counts, or high liver enzymes.
2.you have any other health problems, especially the following:
liver disease such as viral hepatitis or other liver problems
Your doctor will monitor your liver function closely before and during your treatment with Actemra.
HIV or AIDs
tuberculosis
diverticulitis or intestinal ulcers
a low white blood cell count or a low platelet count
diabetes
cancer
heart problems
raised blood pressure
high cholesterol or triglycerides
kidney disease
nerve disease such as neuropathy
3.you are planning to have a vaccination or have recently had a vaccination
Certain types of vaccines should not be given while using Actemra.
4.you are pregnant or plan to become pregnant
Tell your doctor if you are pregnant or plan to become pregnant. Women of childbearing potential should be advised to use adequate contraception during and for several months after treatment with Actemra. Actemra should not be used during pregnancy. However if there is a need to take Actemra when you are pregnant your doctor will discuss the risks and benefits to you and the unborn baby.
5.you are breast-feeding or plan to breast-feed
It is not known whether Actemra passes into breast milk. It is recommended that you discontinue breast-feeding while you are treated with Actemra.
6.you are allergic to any other medicines, foods, dyes or preservatives
If you have not told your doctor about any of the above, tell him or her before you start taking Actemra.
Use in Children
Actemra given as a subcutaneous injection to patients below 18 years with conditions other than pJIA and sJIA has not been studied. Actemra given as a subcutaneous injection in pJIA in children under the age of 2 and sJIA in children under the age of 1 has not been studied. Actemra must not be given to children less than 10 kg.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you have bought from a pharmacy, supermarket or health food shop.
Do not use Actemra with other biological medicines including infliximab, adalimumab, etanercept, anakinra, abatacept, rituximab, certolizumab pegol and golimumab. It is unknown how Actemra interacts with these medicines.
Actemra may interfere with some medicines. These include:
warfarin, a medicine used to prevent blood clots
cyclosporin, a medicine used after organ transplants
some vaccines
atorvastatin and simvastatin, medicines used to reduce cholesterol levels
calcium channel blockers, such as amlodipine, which are used to treat raised blood pressure
theophylline, a medicine used to treat asthma
phenytoin, a medicine used to treat convulsions
benzodiazepines, such as diazepam, which are used to treat anxiety
These medicines may be affected by Actemra or may affect how well the medicine works. You may need to use different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking Actemra.
Ask your doctor or pharmacist if you are not sure about this list of medicines.
How to use Actemra
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
Use Actemra exactly as your doctor has prescribed.
How much to inject
Adult patients with RA and GCA
The recommended dose of Actemra to treat RA or GCA is 162 mg injected once a week. For GCA, your doctor may prescribe a lower dose of 162 mg every 2 weeks.
The syringe is designed to deliver 162 mg per injection when used according to the instructions in this leaflet.
Actemra must be used on the same day of the week. Choose the day of the week that best fits your schedule.
Your doctor will test your blood to help guide your treatment. If you experience certain changes in your blood tests, your doctor may decide to reduce the frequency of dosing to 162 mg every 2 weeks.
For RA, Actemra is usually given in combination with methotrexate (MTX). However you may use Actemra on its own if your doctor determines that initial treatment with MTX is inappropriate or unsuccessful.
For GCA, Actemra is initially given in combination with a glucocorticoid medicine (such as prednisone). Over the period of treatment, depending on your response to Actemra, your doctor will adjust the dose of the glucocorticoid with the aim to reduce it over time.
For children and adolescents with pJIA (aged 2 and over)
The usual dose of Actemra depends on the patient’s weight.
If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled syringe), once every 3 weeks
If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled syringe), once every 2 weeks
For children and adolescents with sJIA (aged 1 year and over)
The usual dose of Actemra depends on the patient’s weight.
If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled syringe) once every 2 weeks
If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled syringe) once every week
Actemra must not be given to children less than 10 kg.
The increasing body weight of a child initially under 30kg should be checked regularly. This is because there is a risk of underdose for this medicine if the frequency of administration does not increase from every 3 weeks to every 2 weeks for pJIA patients (or every 2 weeks to once every week for sJIA patients) as the child grows from under to over 30 kg body weight.
How to inject Actemra
Actemra is administered by subcutaneous injection. This means it is injected with a short needle into the fatty tissue just under the skin.
Serious allergic reactions can occur with Actemra injections.
At least the first injection of Actemra will be given under the supervision of your healthcare provider in a healthcare facility that can manage these reactions. After your first injection, your doctor may discuss with you whether it would be appropriate for you to inject the next Actemra injection yourself at home, in which case, you or a caregiver would be instructed on how to give the injection and what to do if you experience symptoms of an allergic reaction.
Directions for self-injection
You should read these directions from beginning to end before starting to inject so that you are familiar with each step of the procedure. These instructions must be carefully followed. Consult with your healthcare provider if you require further instructions. These instructions do not replace the instructions from your healthcare provider.
Your healthcare provider should show you how to prepare and inject properly before you inject for the first time. Ask them any questions you may have.
Do not attempt to administer an injection until you are sure that you understand how to self-inject.
It is important to remain under your doctor’s care while using Actemra. It is recommended you have someone else present when you self-inject Actemra in case you experience any symptoms of a serious allergic reaction described under While you are using Actemra, Things you must do.
The syringe is for single use only and should be safely discarded after use.
How to inject using the syringe
The syringe components:
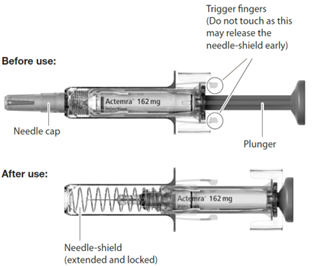
Do not use if the syringe appears to be damaged.
Do not use if the medicine is cloudy, hazy, discoloured or contains particles.
Do not shake the syringe.
Do not try to open the syringe or take it apart.
Do not remove the needle cap until you are ready to inject.
Do not inject through clothing covering the skin.
Do not re-use the same syringe.
Do not touch the syringe trigger fingers as this may damage the syringe.
Gather what you will need:
Included in the pack:
Pre-filled syringe
Not included in the pack:
Alcohol pad
Sterile cotton ball or gauze
Puncture-resistant container (also called a “sharps” container) for safe disposal of the needle cap and used syringe.
Find a well lit, clean, flat surface such as a table.
STEP 1. Visually check the syringe
Take the carton containing the syringes out of the refrigerator and remove one syringe from the carton. Return the remaining syringes in the carton to the refrigerator.
Do not shake.
If there is foam in the medicine, put the syringe back in the carton in the refrigerator for use another time and take a new syringe from the refrigerator.
Visually examine the syringe, as well as the medicine through the viewing window. The injection should be clear and colourless or slightly yellow.
Do not use if the syringe appears to be damaged.
Do not use if the medicine is cloudy, hazy, discoloured or contains particles.
Check the expiration date on the carton and syringe to make sure that it has not expired. The expiry date refers to the last day of that month.
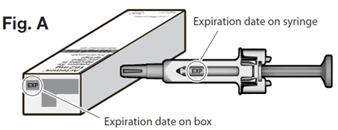
Do not use the syringe if the expiration date has passed.
Do not remove the syringe needle cap until step 5.
STEP 2. Allow the syringe to adjust to room temperature
Place the syringe on a clean flat surface. Allow the syringe to warm up to room temperature which should take 25 to 30 minutes.
Do not warm up the syringe in any other way.
STEP 3. Clean your hands
Wash your hands with soap and water. Cleanliness is vital during the injection procedure.
STEP 4. Choose and prepare an injection site
The recommended injection sites are the front and middle of your thighs and the lower part of the abdomen below the navel (belly button), except for the five centimetre area directly around the navel. If a caregiver is giving the injection, the outer area of the upper arms may also be used.
Use a different place each time you give yourself an injection. The new injection site should be at least 3 centimetres away from your previous injection site.
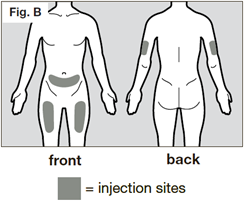
Do not inject into areas that could be irritated by a belt or waistband. Do not inject into moles, scars, bruises, or areas where the skin is tender, red, hard or not intact.
Clean the chosen injection area using the alcohol pad, to reduce the risk of infection. Let the skin dry for approximately 10 seconds. Be sure not to touch the cleaned area prior to the injection. Do not fan or blow on the cleaned area.
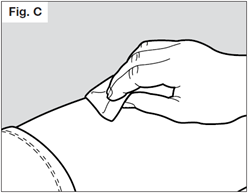
STEP 5. Remove needle cap
Do not hold the syringe by the plunger while removing the needle cap.
Hold the needle shield of the syringe firmly with one hand and pull off the needle cap with the other hand. If you cannot remove the needle cap you should request the help of a caregiver or contact your healthcare provider.
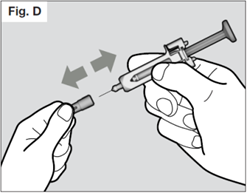
Do not touch the needle or let it touch any surface.
You may see a drop of liquid at the end of the needle. This is normal.
Throw away the needle cap in the sharps container.
Once the needle cap is removed, the syringe should be used immediately, to prevent the medicine from drying out and blocking the needle. If it is not used within 5 minutes, the syringe should be disposed of in the sharps container and a new syringe should be used.
Do not re-attach the needle cap after removal.
STEP 6. Give the injection
Hold the syringe comfortably in your hand. Be careful not to touch the syringe trigger fingers as this may damage the syringe.
To be sure the needle can be inserted correctly under the skin, pinch a fold of loose skin at the clean injection site with your free hand.
Do not hold or push on the plunger while inserting the needle into the skin.
Insert the needle all the way into the pinched skin at an angle between 45° to 90° with a quick, firm action.
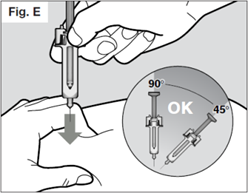
It is important to choose the correct angle to ensure the medication is delivered under the skin (into fatty tissue), otherwise the injection could be painful and the medication may not work.
Then keep the syringe in position and let go of the pinch of skin.
Hold the syringe with two fingers under the flange (or “wings”) and thumb on the plunger. Slowly inject all of the medicine by gently pushing the plunger all the way down.
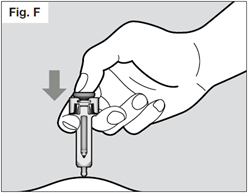
You must press the plunger all the way down to ensure that you get the full dose of medication and to ensure the trigger fingers are completely pushed to the side.
If the plunger is not fully depressed the needle shield will not extend to cover the needle when it is removed.
Once the plunger is pushed all the way down, keep pressing down on the plunger to be sure all of the medicine is injected before taking the needle out of the skin.
Keep pressing down on the plunger while you take the needle out of the skin at the same angle as inserted.
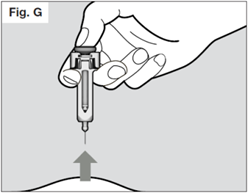
Once the needle is removed completely from the skin, you can release the plunger. The needle will retract allowing the needle shield to protect the needle.
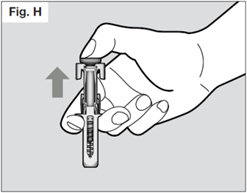
If the needle is still exposed proceed carefully, and place the syringe into the sharps container to avoid injury with the needle (see STEP 7).
If you see drops of blood at the injection site, you can press the sterile cotton ball or gauze over the injection site for approximately 10 seconds.
Do not rub the injection site.
STEP 7. Safely dispose of the syringe
Do not try to re-cap your syringe.
Throw away used syringes in a sharps container.
Ask your healthcare provider or pharmacist for information about where you can get a sharps container or what other types of puncture-resistant containers you can use to safely dispose of your used syringes, if you do not have one.
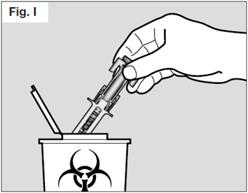
Do not throw away used syringes or the sharps container in household rubbish and do not recycle them.
Dispose of the full container as instructed by your healthcare provider or pharmacist.
Always keep the sharps container out of the reach of children.
How long to use Actemra
The duration of treatment depends on how you are responding to the medicine. Your doctor will discuss this with you.
Continue to use Actemra until your doctor tells you to stop.
If an adult with RA/GCA or a child or adolescent with pJIA or sJIA forgets to use Actemra
It is very important to use Actemra exactly as prescribed by your doctor. Keep track of your next dose.
For once a week Actemra dosing:
If you missed your once a week Actemra dose and you remember within 7 days, you should skip the missed dose.Make sure you inject your next dose normally on the next scheduled day.
For example, if you forget your scheduled dose on Monday but you remember on Wednesday, you should skip your missed dose and inject the next dose as you would normally on the following Monday.
Do not give yourself two injections to make up for the injection that you missed.
If it has been more than 7 days since your missed dose of Actemra, please contact your doctor for advice.
If you are not sure when to inject your next dose, contact your doctor for advice
For fortnightly or every three week Actemra dosing:
If you missed your fortnightly or every three week Actemra dose and you have remembered within 7 days of the dose you missed, you should inject the missed dose as soon as possible. Inject the next dose as you would on the next scheduled day.
Do not give yourself two injections to make up for the injection that you missed.
If it has been more than 7 days since your missed dose, contact your your doctor for advice.
If you are not sure when to inject your next dose, contact your doctor for advice.
Overdose
If you think that you or anyone else may have used too much Actemra, immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at your nearest hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
While you are using Actemra
Things you must do
When using Actemra, there is a risk of serious allergic reaction which needs immediate medical attention. Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you experience symptoms of a serious allergic reaction during or after using Actemra such as;
chest tightness, wheezing or difficulty breathing
severe dizziness or light-headedness
swelling of the face, lips, tongue, throat or other parts of the body with difficulty breathing
rash, itching or hives on the skin.
The reaction can occur even after multiple doses of Actemra. If you have experienced any allergic reaction symptoms after using Actemra, do not take the next dose until you have informed your doctor AND your doctor has told you it is safe to take the next dose.
Tell your doctor immediately if you develop an infection or have symptoms of an infection while you are using Actemra. Signs of an infection, with or without fever include:
sweating or chills,
feeling very tired
cough
shortness of breath
muscle aches
weight loss
warm, red, or painful skin or sores on your body
blood in phlegm
diarrhoea or stomach ache
persistent headaches.
Tell your doctor immediately if you develop severe blisters and bleeding in the lips, eyes, mouth, nose and genitals while you are using Actemra.
When using immunosuppressive medication (lowers resistance to disease): such as Actemra, there is an increased risk of developing skin cancer (melanoma and non-melanoma). Regular skin examination is recommended if you are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using sunscreen with a high protection factor.
Tell all doctors, dentists and pharmacists who are treating you that you are using Actemra.
Tell your doctor if you become pregnant while using Actemra.
Tell your doctor if you are breast-feeding while using Actemra.
Tell your doctor if you feel Actemra is not helping your condition.
Be sure to keep all of your appointments with your doctor so that your progress can be checked.
Your doctor will perform blood tests at regular intervals during your treatment to determine if you have low white blood cell or platelet counts, or high liver enzymes or cholesterol.
Things you must not do
You should not breast-feed your infant during treatment with Actemra.
It is not known whether Actemra crosses into human milk.
Do not take any other medicines whether they require a prescription or not without first telling your doctor or consulting a pharmacist.
Things to be careful of
Be careful driving or operating machinery until you know how Actemra affects you.
Actemra has not been shown to impair the ability to drive or operate machinery. However if you experience dizziness, a reported side effect, then you should not drive or operate machinery until it has resolved.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using Actemra.
Actemra helps many patients with RA, GCA, pJIA and sJIA but it may have unwanted side effects.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
chest tightness, wheezing or difficulty breathing
severe dizziness or light-headedness
swelling of the face, lips, tongue, throat or other parts of your body with difficulty breathing
rash, itching or hives on the skin
signs of an infection with or without fever
signs of tears (perforation) of the stomach or intestines such as fever and pain in the stomach area, vomiting blood or material that looks like coffee grounds, bleeding from your rectum, and a change in your bowel habits
severe blisters and bleeding in the lips, eyes, mouth, nose and genitals.
signsof liver disease, hepatitis and/or jaundice including:nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, light coloured bowel motions, dark coloured urine.
These are serious side effects. You may need urgent medical attention. Serious side effects are rare.
Tell your doctor if you notice any of the following and they worry you:
high blood pressure (symptoms may include headache, dizziness, ringing in the ears)
headache
upper respiratory tract infection such as a common cold or sinus infection (cough, blocked or runny nose, sore throat)
dizziness
nausea or indigestion
stomach pain
constipation
diarrhoea
back pain
cold sores
mouth or skin blisters
mouth ulcers
skin infection (redness, pain and/or swelling)
injection site reactions, such as pain, redness and itch
low white blood cell and platelet counts shown by blood tests
raised blood fat (cholesterol) levels
These are the more common side effects of Actemra. Mostly these are mild.
Side effects in children and adolescents with pJIA and sJIA
Side effects in children and adolescents with pJIA and sJIA are generally similar to those in adults. Some side effects are seen more often in children and adolescents: inflamed nose and throat, headache, feeling sick (nausea) and lower white blood cell counts.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don’t understand anything in this list.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Storage
Store in a refrigerator (2°C to 8°C). Do not freeze.
Store the syringes in the carton to protect them from light and to keep them dry.
Once removed from the refrigerator, Actemra must be used within 8 hours and should not be kept above 30°C.
Do not use Actemra after the expiry date which is stated on the carton and syringe labels after ‘EXP’. The expiry date refers to the last day of that month.
Disposal
The syringe is intended for single use only and must be discarded after the injection.
Dispose of the syringes in a sharps container as instructed by your doctor, nurse or pharmacist.
Do not put the used syringes in your normal household rubbish.
If your doctor tells you to stop using Actemra, or the product has passed its expiry date, ask your pharmacist what to do with any medicine that is left over.
Availability
Actemra is available as a pre-filled syringe (162mg/0.9mL) in packs of 1 and 4 syringes.
Actemra is also available as a concentrated solution for intravenous infusion.
What Actemra looks like
Actemra is a clear to opalescent, colourless to pale yellow solution.
Ingredients
Active ingredient – tocilizumab (rch)
Inactive ingredients
polysorbate 80
histidine
histidine hydrochloride
arginine
arginine hydrochloride
methionine
water for injections
Distributor
Actemra is distributed in Australia by:
Roche Products Pty Limited
ABN 70 000 132 865
Level 8, 30-34 Hickson Road
Sydney NSW 2000 Australia
Medical enquiries: 1800 233 950
Please check with your pharmacist for the latest Consumer Medicine Information.
Australian Registration Numbers
162mg/0.9mL pre-filled syringe AUST R 234034
This leaflet was prepared on 1 April 2020.












