Home » Health News »
Researchers create a new window on leading genetic cause of Alzheimers
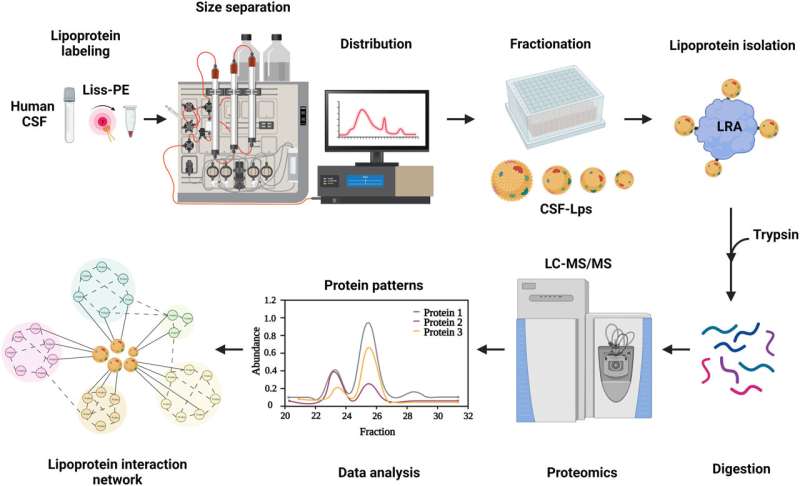
Scientists have created a method to detect key fat-filled particles known as lipoproteins in the central nervous system, opening a new view into the workings of the brain.
Researchers found that the particles in our nervous system, molecular cousins of the well-known HDL, or “good cholesterol” particles in our bloodstream, are much more diverse than previously thought. Researchers detected more than 300 different proteins associated with the particles, far more than the 16 known previously, that fall into at least 10 different families. These particles are rich in proteins that affect wound healing, the immune response, and the creation and nurturing of brain cells called neurons that are important for cognitive function.
The most common protein on the particles is apolipoprotein E, better known as APOE. Of the three commonly studied forms of APOE, the form known as APOE4 puts people at higher risk of Alzheimer’s disease. One copy of the APOE4 gene makes a person approximately four times as likely to develop dementia; a person with two copies is about 12 times more vulnerable.
The results were published Aug. 30 in the journal Science Advances. The leader of the study is John Melchior, a protein biochemist at the Department of Energy’s Pacific Northwest National Laboratory. Melchior is a leader in lipoprotein research—and a carrier of two copies of the APOE4 gene, adding a personal incentive to his work to understand the protein’s action in the nervous system.
“We’ve known for a long time that in the nervous system, APOE is the primary protein on these particles calling the shots. But we don’t know much more beyond that. Our technology opens the door to learning more,” said Melchior.
“What the heck is APOE4 doing? That’s the big question. Why does one form translate to less risk for dementia while a slightly different form confers significant risk? Our technology brings us one step toward more answers,” he added.
APOE: Bringing fats and proteins together
Lipoproteins are best known for their work in the circulatory system, where they transport fat and cholesterol. It’s easy for scientists to detect HDL and LDL, known to many as “good cholesterol” and “bad cholesterol,” in the bloodstream because the molecules there are plentiful.
But lipoproteins in the nervous system are much scanter, present at less than 1 percent of the concentration in blood. Their actions, even their presence, in the nervous system have been a mystery. Scientists know that these balls of fat and protein wrapped together travel in the bloodstream and carry out all sorts of important functions, like shuttling cholesterol and nutrients.
On the particles in the central nervous system, APOE reigns supreme, serving as a scaffold to hold lipids and other proteins together. It also transports these nutrient-rich lipids and collections of molecular collaborators—groupings of proteins on its surface—throughout the nervous system to perform their tasks.
The proteins are specialized tools that can do things like repair cells, turn genes on or off, or regulate amyloid beta processing, a well-known molecule related to the development of dementia. The work suggests that APOE may bring these tools together on different particles to deliver them where needed.
But something is more likely to go wrong in people with one or two copies of APOE4, leading to dementia. Scientists don’t know what.
Scientists suspect APOE4 of playing a role in other neurologic conditions such as Parkinson’s and Huntington’s diseases, multiple sclerosis, amyotrophic lateral sclerosis and even traumatic brain injury.
Because lipoproteins are less common in the nervous system, researchers either need an impossibly large amount of the cerebrospinal fluid to study them—or scientists develop a new way to detect the rare molecules. That’s what Melchior’s team did, creating a new fluorescent technology to tag lipoproteins in spinal fluid.
The team studied just one-third of a milliliter of spinal fluid—much less fluid than in a raindrop—and discovered 303 different proteins across the particle families using mass spectrometry. Most had never been detected on these particles in the nervous system before.
A solid start and next steps: Lipoproteins and neurological diseases
“Now comes the fun part,” said Melchior. “We want to open up our technology to clinicians to learn more about what’s happening in Alzheimer’s disease and possibly other conditions like multiple sclerosis and Parkinson’s disease.
“There are existing cerebrospinal fluid samples sitting in freezers, and we have a new way to analyze them. We’d love to work together with other research teams to investigate them. The sooner we can start profiling lipoproteins in these conditions, the sooner we can understand more about their role in disease pathology and identify targets for treatments,” added Melchior, who holds joint appointments at Oregon Health & Science University and the University of Cincinnati.
More information:
Nathaniel J. Merrill et al, Human cerebrospinal fluid contains diverse lipoprotein subspecies enriched in proteins implicated in central nervous system health, Science Advances (2023). DOI: 10.1126/sciadv.adi5571
Journal information:
Science Advances
Source: Read Full Article


