Home » Health News »
Superior immune response in vaccinated after infection with Omicron compared to unvaccinated
In a recent study posted to the medRxiv* preprint server, researchers evaluated the cross-reactivity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant-triggered neutralization and fragment crystallizable (Fc) effector functions between coronavirus disease 2019 (COVID-19)-vaccinated and unvaccinated individuals in South Africa.

Study: SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated individuals. Image Credit: anushkaniroshan/Shutterstock
The first cases of the SARS-CoV-2 Omicron (B.1.1.529) variant were reported from South Africa to the World Health Organization (WHO) in November 2021. Soon after, it caused the fourth wave of the COVID-19 pandemic in South Africa. Omicron is characterized by more than 30 mutations in its spike (S) protein region compared to other SARS-CoV-2 variants, many of which enhance the immune evasion capacity of the Omicron variant in the host.
Studies have established that the mutations present in the SARS-CoV-2 Omicron variant grant the capacity to escape the natural infection- or vaccination-induced neutralizing antibodies to a large extent. However, sufficient information regarding the cross-reactivity of Omicron-triggered humoral immune responses to other SARS-CoV-2 variants of concern (VOCs) is not yet available.
About the study
In the current study, the researchers evaluated the levels of binding antibodies, antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), and neutralization induced by Omicron against other SARS-CoV-2 VOCs such as Delta, D614G, Beta, and C.1.2 in COVID-19 vaccinated and unvaccinated individuals using the enzyme-linked immunosorbent assay (ELISA).
The team examined plasma from seven COVID-19 vaccinated and 20 unvaccinated subjects infected during the SARS-CoV-2 Omicron wave in South Africa to determine the cross-reactivity against other VOCs. The plasma from the 27 subjects was procured between November 25 and December 20, 2021, when the Omicron variant was responsible for more than 90% of COVID-19 cases in South Africa.
Of the plasma collected from the 27 individuals, the plasma from the seven vaccinated individuals had matching nasal swabs demonstrating confirmed Omicron BA.1 infection by sequencing. The remaining 20 subjects were unvaccinated with no prior symptomatic SARS-CoV-2 history.
Results
The results indicated that binding antibody titers were the highest against Omicron in SARS-CoV-2 unvaccinated individuals with Omicron infection and were detectable among all participants. Statistically significant 1.7, 1.8, and 2.2-times reductions in binding towards Delta, Beta, and ancestral D614G were observed in this cohort.
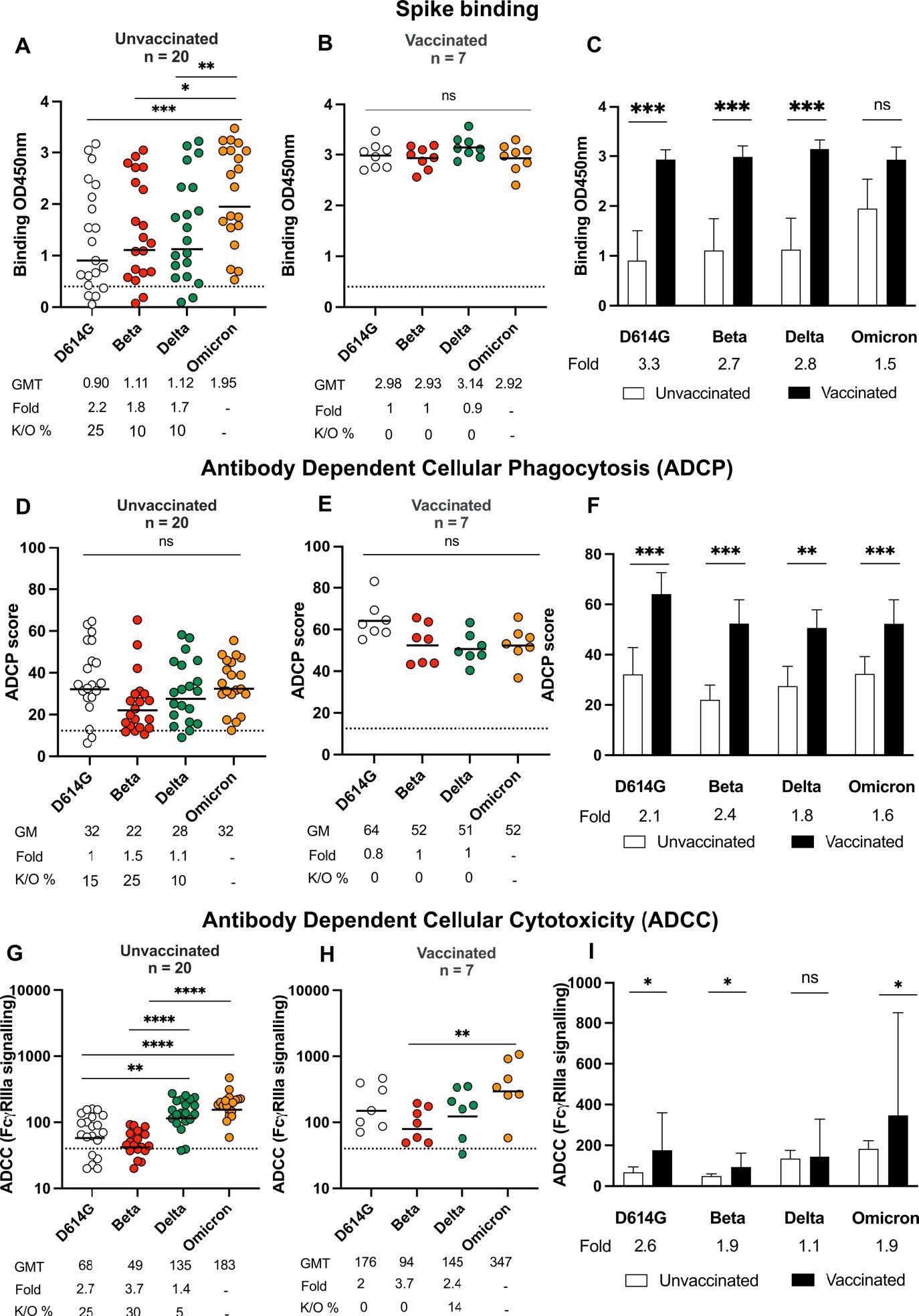
Binding and Fc effector function elicited by Omicron infection is crossreactive against several variants of concern Antibody binding measured by ELISA in (A) unvaccinated individuals (n =20) or (B) individuals vaccinated with either one dose of Ad26.CoV.2S or two doses of BNT162b2 (n=7) and infected by Omicron against D614G, Beta, Delta and Omicron spike proteins. (C) Bars show geometric mean binding titers for vaccinated (black) and unvaccinated (white) individuals against variants of concern. Antibody-dependent cellular phagocytosis (ADCP) of (D) unvaccinated and (E) vaccinated individuals is represented as the percentage of monocytic cells that take up spike coated beads (D614G, Beta, Delta and Omicron) multiplied by their geometric mean fluorescence intensity (MFI). (F) Bars show geometric mean ADCP scores for vaccinated (black) and unvaccinated (white) individuals against variants of concern. Antibody-dependent cellular cytotoxicity (ADCC) in (G) unvaccinated and (H) vaccinated individuals shown as relative light units (RLU) signaling through FcgRIIIa expressing cells. (I) Bars show geometric mean activity for vaccinated (black) and unvaccinated (white) individuals against variants of concern. All data are representative of two independent experiments. For dot plots, lines indicate geometric mean titer (GMT) also represented below the plot with fold decrease and knock-out (K/O) of activity for other variants as a percentage relative to Omicron. Dotted lines indicate the limit of detection of the particular assay. For bar charts, bars indicate median of function, with error bars showing standard deviations with fold decreases relative to vaccinated individuals, indicated below the plot. Statistical significance across variants is shown by Friedman test with Dunn’s correction and between vaccinated and unvaccinated samples by the Mann Whitney test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 and ns = non-significant.
In all unvaccinated Omicron-infected subjects, ADCP against Omicron was detectable whereas, a less than two-times reduction in ADCP activity was observed against the Delta, Beta, and D614G variants. Further, ADCC activity in COVID-19 unvaccinated subjects was significantly reduced against the SARS-CoV-2 Beta and D614G variants.
Nonetheless, Omicron-stimulated neutralization demonstrated 20- to 43-times reductions in titers of cross-reactivity towards SARS-CoV-2 variants such as C.1.2, Beta, Delta, and D614G in these subjects. Hence, indicating the susceptibility of Omicron-infected unvaccinated individuals to reinfection with other emerging or circulating VOCs.
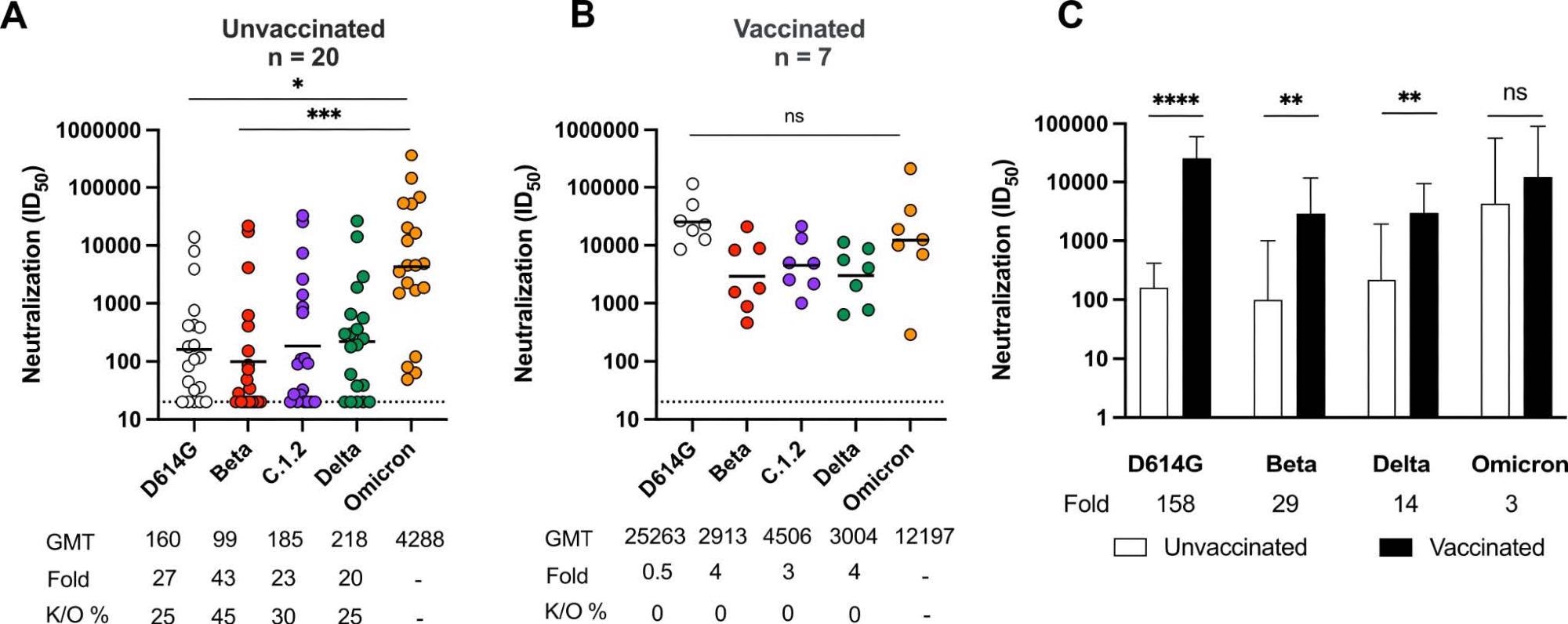
Omicron triggers cross-variant neutralizing antibodies which are broadened by vaccination Neutralization titer (ID50) of Omicron-infected plasma against D614G, Beta, C.1.2, Delta and Omicron pseudoviruses shown for (A) unvaccinated individuals (n=20) or (B) individuals vaccinated with either one dose of Ad26.CoV.2S or two doses of BNT162b2 (n=7). Lines indicate geometric mean titer (GMT) also represented below the plot with fold decrease and knockout (K/O) of activity for other variants as a percentage relative to Omicron. Dotted lines indicate the limit of detection of the assay. Statistical significance across variants is shown by Friedman test with Dunns correction. (C) Bars show geometric mean neutralization titers for vaccinated (black) and unvaccinated (white) individuals against variants of concern with error bars showing standard deviations with fold decreases relative to vaccinated individuals indicated below the plot. Statistical significance between vaccinated and unvaccinated samples by the Mann Whitney test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 and ns = non-significant.
By contrast, the COVID-19-vaccinated individuals infected with Omicron had high levels of binding and Fc effector function antibodies targeting Omicron relative to the unvaccinated subjects.
In detail, the binding antibody titers in these cohorts demonstrated higher cross-reactivity to all SARS-CoV-2 variants than the unvaccinated lots. The SARS-CoV-2 vaccinated cohorts showed negligible reductions in ADCP against Delta, Beta, and D614G relative to the Omicron and demonstrated activity towards all variants evaluated. The ADCC titers against Beta, ancestral D614G were higher in previously vaccinated Omicron-infected individuals compared to the unvaccinated group. However, in the case of the Delta variant, comparable levels of ADCC were observed in both groups.
Further, neutralizing antibodies in vaccinated individuals with breakthrough Omicron infection demonstrated enhanced cross-neutralization of all SARS-CoV-2 VOCs evaluated.
Conclusions
The study findings show that although Omicron infection is a potent booster of immunity, it cannot be considered superior to the existing COVID-19 vaccine regimens for priming an individual without SARS-CoV-2 history.
The study indicates that Omicron does not produce cross-neutralizing responses in unvaccinated individuals, whereas it boosted humoral immune responses in vaccinated subjects. Further, it demonstrates that Omicron-infection induces similar neutralization titers regardless of the vaccination status while evaluating the SARS-CoV-2 strain-specific responses. Hence, the study offers valuable information for designing Omicron-based second-generation vaccines.
Overall, the study provides deep insights regarding the utility of Omicron as an immunogen, the probability for reinfection in individuals not vaccinated against COVID-19, and vaccination-enhanced cross-reactive responses.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated individuals, Simone I Richardson, Vimbai Sharon Madzorera, Holly Spencer, Nelia P Manamela, Mieke van der Mescht, Bronwen E Lambson, Brent Oosthuysen, Frances Ayres, Zanele Makhado, Thandeka Moyo-Gwete, Nonkululeko Mzindle, Thopisang Motlou, Amy Strydom, Adriano Mendes, Houriiyah Tegally, Zelda de Beer, Talita Roma de Villiers, Annie Bodenstein, Gretha van den Berg, Marietjie Venter, Tulio de Oliviera, Veronica Ueckermann, Theresa M Rossouw, Michael T Boswell, Penny L Moore, medRxiv, 2022.02.10.22270789; doi: https://doi.org/10.1101/2022.02.10.22270789, https://www.medrxiv.org/content/10.1101/2022.02.10.22270789v1
Posted in: Medical Research News | Disease/Infection News
Tags: ADCC, Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytotoxicity, Enzyme, Fluorescence, Immune Response, immunity, Knockout, Omicron, Pandemic, Phagocytosis, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article



